Metals form strong bonds because they are elements from the part of the periodic table where positive nuclei line up in a particular way. Strong bonds form between atoms thanks to the negative ions that slot in and ‘cement’ the atoms together in a regular arrangement. Breaking these bonds can require strong external forces in the form of heat or strong chemicals such as hydrochloric acid.
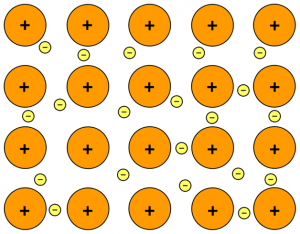
Characteristics of Bonds
The strong, regular bonds within metals give these substances many of the distinctive properties we associate with metals, such as smoothness and the ability to conduct heat or electricity. Many metals are malleable, and some are very strong. However, not all metals are like this. If you take sodium, which is an alkali metal, it is very soft at room temperature and will spontaneously combust and oxidize. In other words, if you put it in the air, it will burst into flame and combine with oxygen to form sodium oxide.
Metals such as iron are strong and solid at room temperature, although iron will react slowly with oxygen in the presence of water as well. Rust is the result, a hydrating combination of iron and oxygen known as ferric oxide.
Uses and Applications
Metals have many applications in design, crafts, and industry. For those who want to work with metals in designing all kinds of objects, there are many courses available. If this appeals, it would be worth looking at this league table in The Guardian to see how courses with metals at different institutions compare.
Metals are also frequently used in construction, as support structures such as steel girders give a building extra strength and stability. When it comes to roofs, lead is a popular material for flashing, as it is malleable and slow to oxidize. Roof sealant also needs to be stable and non-reactive to do its job properly. If you want to find out more about roof sealant, then it would be worthwhile contacting a trusted roof sealant supplier where you can discover more about options available.
Metals are an integral part of daily life. Without metals, it would be difficult to imagine what we would make engines or computer chips from. Many metals are durable and long-lasting, and these qualities are due to how their composite atoms behave at the chemical level.

